SILKTOE
Features
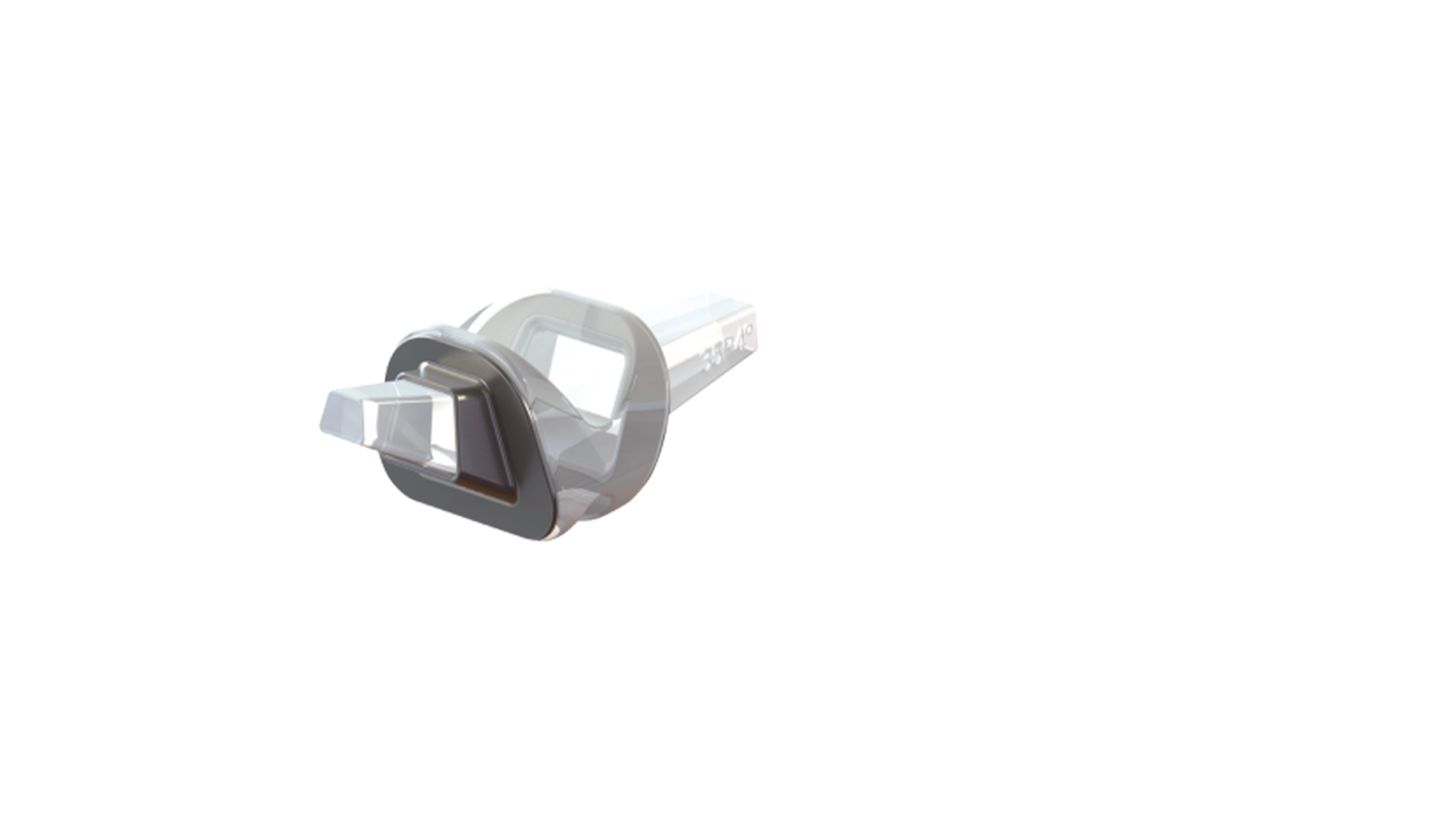
- Trapezoidal stem ensures the device fits with patient anatomy
- Rectangular section to aid with intramedullary metatarsal canal adaptation
- Elastomeric silicone material for flexibility
- Stability support
SILKTOE Arthroplasty System
This product is for use in all severe forms of arthritis that have resulted bone degradation and damage to bone surfaces. This includes rheumatoid arthritis, arthritis in the abducted hallux valgus, painful/unstable joints, ankylosed or limited joints, and instances where joint surfaces have been destroyed. The SILKTOE system contains:
- A silicone elastomer spacer in a range of sizes.
- Titanium allow grommets.
- Comprehensive instrumentation set – sterilisation box, multifunctional Silktoe handle, cutting guides, trial sizers, impactors, metatarsal and phalangeal rasps.
- Sliding unit.
During metatarsophalangeal joint arthroplasty, the device’s silicone body is used to replace the MTP1 joint in the foot. The created interface allows natural dorsiflexion movement to be restored in the patient. Stability is ensured through the grommets, which are positioned at precise intervals above the stem extensions of the SILKTOE. These support the spacer with the aid of surface reliefs at the point where the bone interfaces with the device. The hinged joint then prevents contact between grommets during toe movements.
Interested in the SILKTOE?

BRM Extremities is a company dedicated to the design and production of orthopaedic devices for use in the extremities, both upper and lower. They combine modern technologies with extensive medical knowledge to support public and private healthcare professionals. The mission of BRM Extremities is to create a catalogue of quality medical devices that can respond to the increasing demands of the orthopaedic sector. The company is certified to ISO 13485: 2016 and ISO 9001: 2015 standards and is aware of relevant device regulations in the FDA, CE and ANVISA fields.
Frequently Asked Questions
As with any orthopaedic implant, considerations must be made regarding the patient and their suitability for the procedure. The risks associated with implanting the SILKTOE product include:
- Active sepsis.
- Child patients.
- Deficiency in the circulatory system, skin, or bones of the patient.
- Psychologically unsuitable patient.
There is no defined expiry date for the SILKTOE system. However, implants and instrumentation must be inspected before use. This means checking that there are no visible signs of wear, breakage, or deformation. Sterilisation will also be necessary prior to an arthroplasty procedure.