

PANTERA® – Proximal Humerus Plate System
Pantera is the most advanced proximal humerus fracture plate on the market. Features include:
- Suture clips for easy repair of the rotator cuff.
- Advanced options for cross screw fixation to resist humeral head collapse.
- Lesser tuberosity fixation options.
Pantera Proximal Humerus Plate System
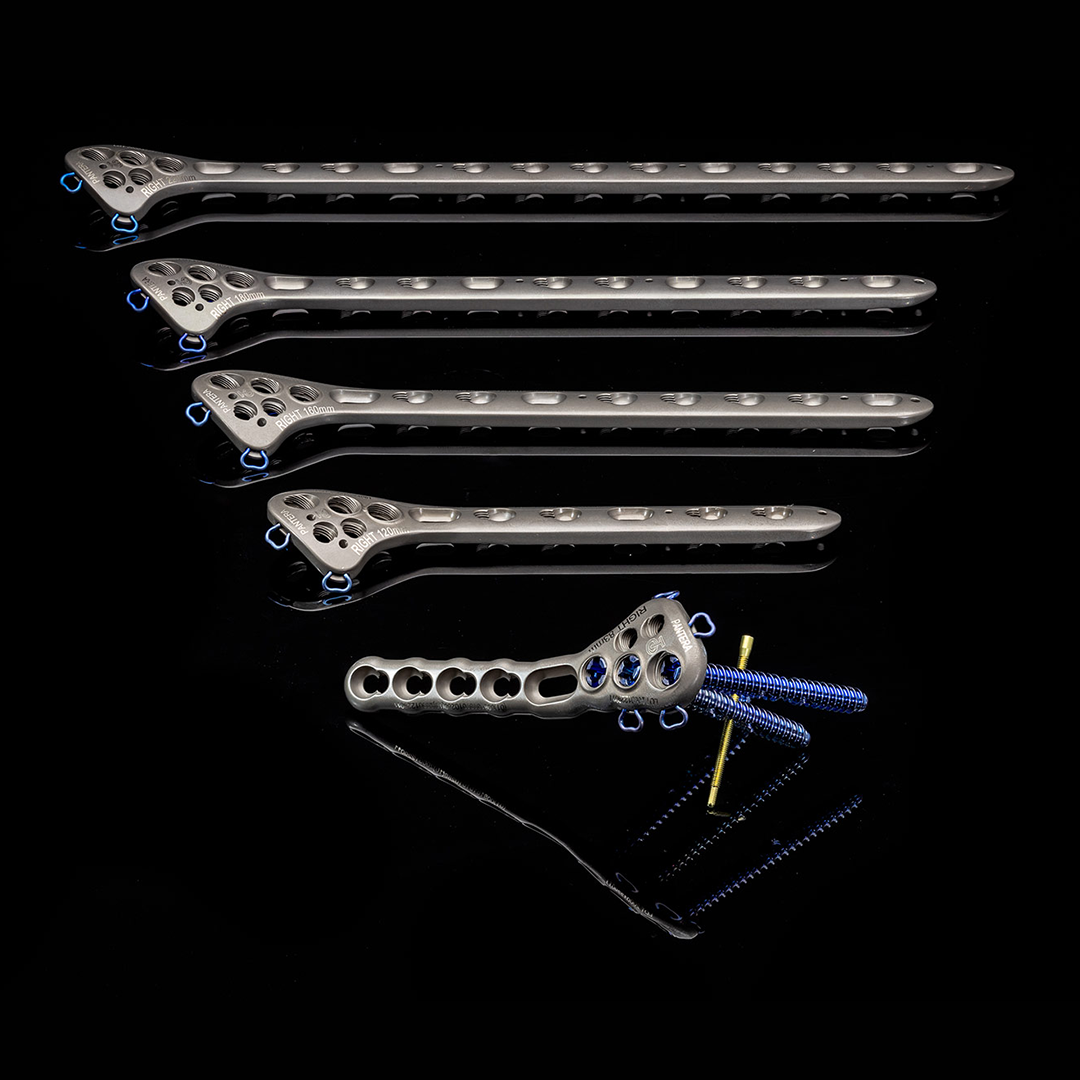
The Pantera Proximal Humerus Plate system contains bone plates for the repair of proximal humerus fractures. It is designed to help surgeons restore fracture stability even in cases where bones are severely osteoporotic. Weak bone density can increase the risk of upper arm fractures as a result of trauma. As falls are one of the most common causes of arm bone fractures, the Pantera system has a wide application. It’s indicated for proximal humerus fractures, fracture dislocations, osteotomies, and non-unions.
Plate fixation is used to treat proximal humerus fractures and restore shoulder movement. It does this through anatomic reduction and stabilization of the humeral head complex. Screws should be strategically placed so they make use of the stronger aspects of the proximal humeral head (medial and dorsal). Locking plates protect the fracture site and facilitate normal healing, all while causing minimal soft tissue dissection.
System Contents
The Pantera Proximal Humerus System includes:
- Titanium alloy plate – available in 73mm, 83mm, 120mm, 160mm, 180mm and 220mm lengths, as well as left and right configurations.
- 5.2mm cannulated and non-cannulated posts
- 3.5mm locking and non-locking cortical screws
- Post cap screws
- K-wires – not for internal implantation
- Specialized instrumentation
Patents: U.S. 8,182,485; U.S. 8,361,075; U.S. 8,574,234
Benefits of the PANTERA proximal Humerus Plating
- Resistant to Varus Collapse
- Low Impingement Risk
- Rigid Fixation of Lesser Tuberosity
- Ease of Access for Soft Tissue Attachments
The suture clips, which are unique to Pantera, make it easy to repair rotator cuff tears associated with humerus fractures. They act as plate extensions, allowing for the reconstruction of soft tissues and comminuted bone. This means suture clips can buttress the comminuted cortical bone of the tuberosities.
Pantera is a low-profile plate which can also be used as a conventional locking plate. This has biomechanical benefits, such as reduced wound healing complications, high rigidity, and being able to provide under high loading conditions.
Advanced features of the system, such as crossing screw fixation, allow surgeons to minimize humeral head collapse. The cross-screw system is also ideal for providing fixation of the lesser tuberosity. This makes Pantera the only plating system addressing lesser tuberosity fracture fixation. This is a rare type of injury that involves fractures to multiple parts of the proximal humerus due to shoulder joint dislocation. The system’s cross screw feature can enhance soft bone fixation up to five times better than conventional screws.
INTERESTED IN PANTERA?
If you would like more information regarding Toby Orthopaedics’ PANTERA system
Interested in the PANTERA® – Proximal Humerus Plate System ?
Most Advanced Proximal Humeras Plate on the Market

Available in a wide range of lengths

Patented Cross Element Technology
Related Products

Founded in 2003, Toby Orthopaedics began as an idea-house inspired by orthopaedic dilemmas in the operating room. Since then, it has evolved to become an industry leader in medical and surgical devices. Today Toby is focused on one goal – to enhance the wellness and quality of life of patients from around the world using innovative movement solutions. This drives Toby Orthopaedics to seek constant improvement through relentless research, placing them at the forefront of the healthcare design industry.
Toby’s musculoskeletal solutions are uniquely designed and engineered to treat a range of bone structures. The company is based Miami, Florida.
Frequently Asked Questions
There are few cases where it is necessary to remove plate implants, especially larger ones like those used in proximal humerus fractures. Most surgical professionals won’t consider plate removal to be ‘routine care’. Plate implants can typically remain inside the body without causing any harm.
When plate fixation results in long-term complications like pain, irritation or infection, it can create the need for plate removal.
Proximal humerus fractures will usually be incurred due to shoulder joint trauma. This can be caused by impact to the upper arm, either directly through a fall or indirectly the transfer of force to the shoulder joint. This is compounded in instances where patients have low bone strength. Diseases like osteoporosis and certain kinds of tumors can be factors in bone weakening.
Osteoporosis is a disease that decreases bone strength, making them more susceptible to breakages. Osteoporotic bones are those affected by osteoporosis. The disease can develop when bone structure or quality undergoes changes, or when bone mass or density falls. There are many risk factors associated with the development of osteoporosis. The most contributing are age, lifestyle, diet, sex, and other medical conditions.
All components and specialized instruments in the Proximal Humerus Plate System are supplied non-sterile. Resultantly, healthcare facilities must subject the system to sterilization. Moist heat sterilization is the recommended method, which can be done using any suitable container. The system should be wrapped in accordance with 510(k) clearance and sterilized in the following process:
- Type – Pre-vacuum
- Minimum Temperature – 135ºC
- Full Cycle Time – 3 minutes
- Drying Time – 20 minutes
Following sterilization, the plate system should be stored in a cool dry place away from direct sunlight. Then prior to use, system components should be inspected for signs of damage, tampering, or contamination.

