Threaded Hand Nail
Features
- Stable fracture repair
- Thread design balances canal fill and lower insertion torque
- Controlled translation
- Net Zero Compression
Fixation of osseous injuries
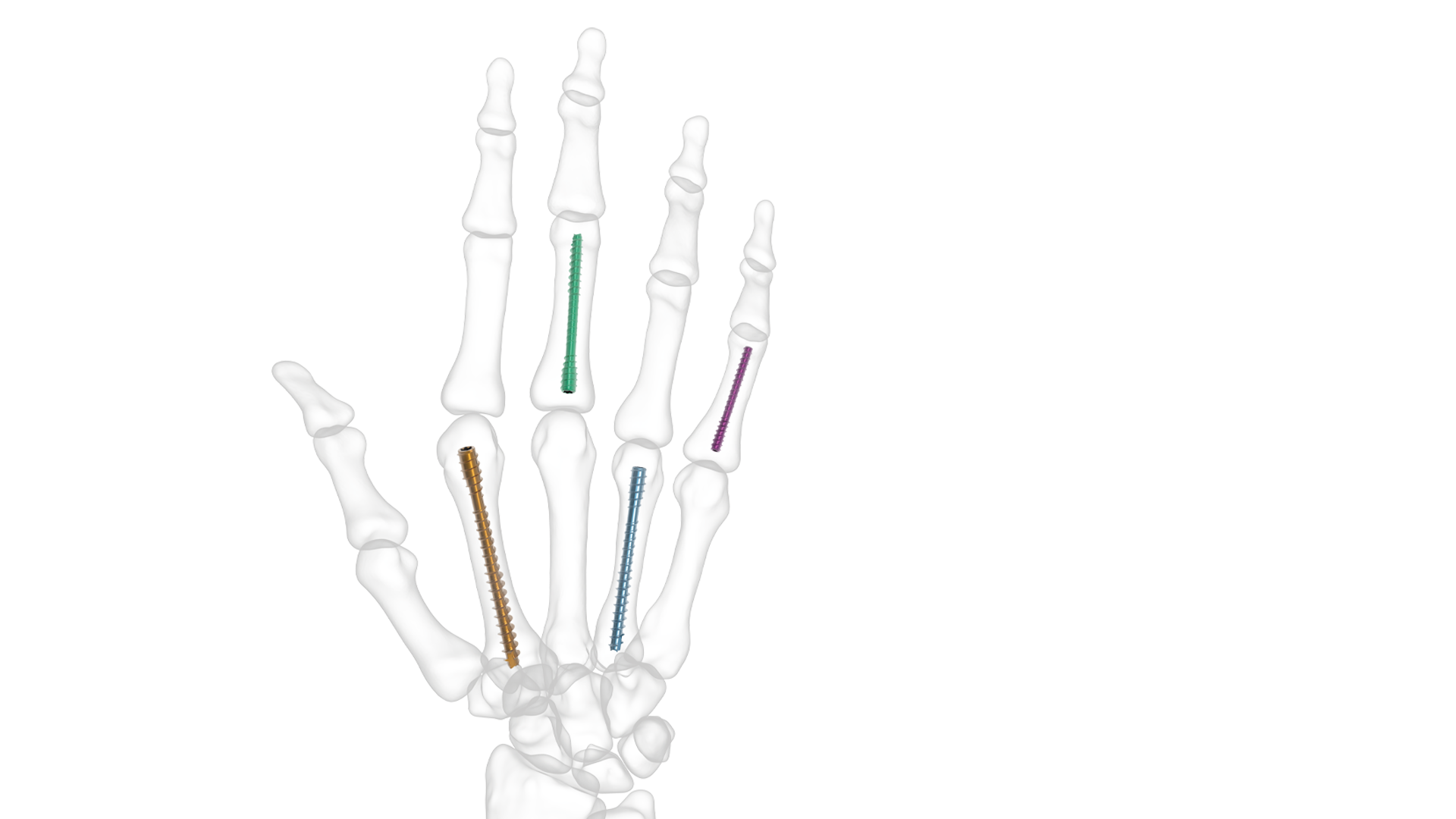
The Threaded Hand Nail System from Skeletal Dynamics is intended for the internal fixation of hand fractures, osseous fragments, osteotomies, and arthrodesis of small joints in the hand. This product sees use in various types of injury at locations throughout the hand. This includes key joints such as the STT joint and for the fixation of carpometacarpal fractures. Surgeons are free to select the size of nails that they deem appropriate for patient anatomy in any given case, bringing an added level of flexibility in application.
System contents
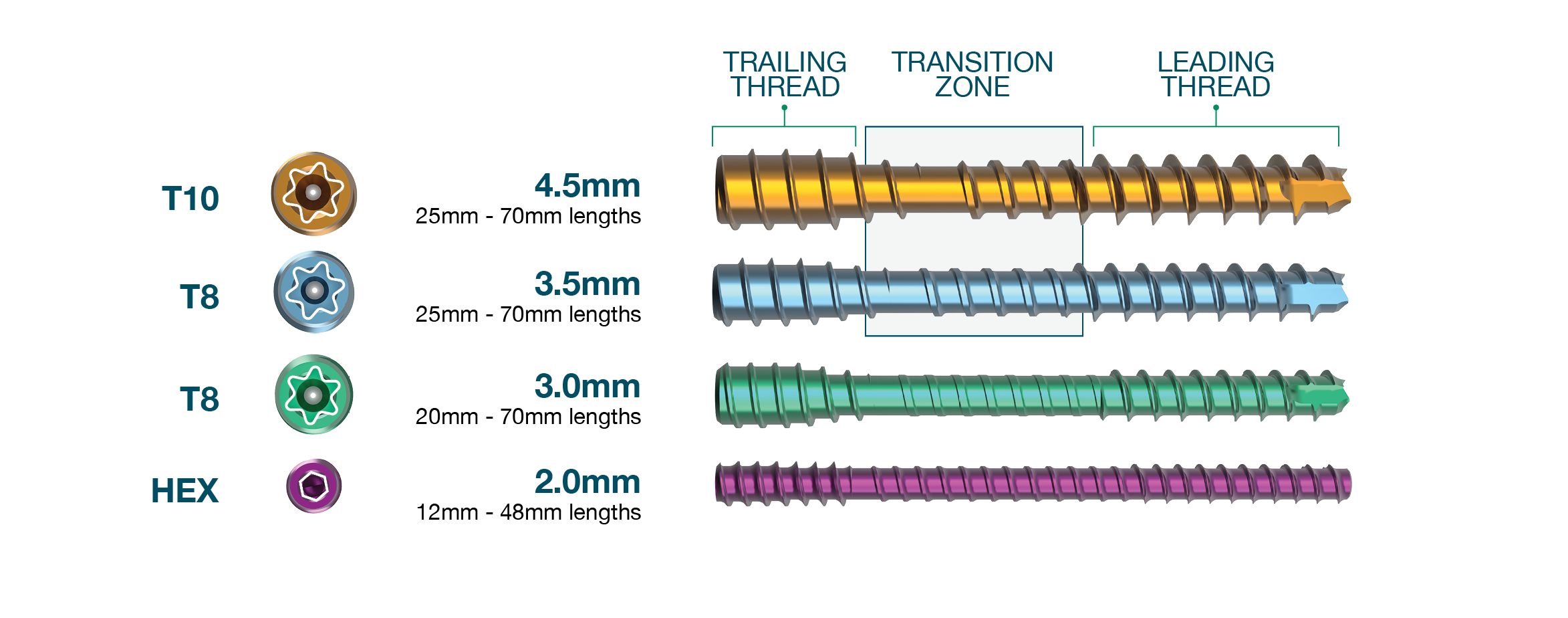
The following threaded hand nails which are contained in the system are made from medical grade titanium alloy (ASTM F-136). The specifications are:
- 2.0mm non-cannulated threaded nail in lengths from 12mm to 28mm (2mm increments).
- 2.0mm non-cannulated threaded nail in lengths from 32mm to 48mm (4mm increments).
- 3.0mm cannulated threaded nail in lengths from 20mm to 70mm (5mm increments).
- 3.5mm cannulated threaded nail in lengths from 25mm to 70mm (5mm increments).
- 4.5mm cannulated threaded nail in lengths from 25mm to 70mm (5mm increments).
In addition to the nails themselves, the Hand Trauma Threaded Nail System contains hand surgery instruments that are specific to the types of procedures that the system is intended. This includes:
- Single Trocar K-Wire (1.4mm x 203mm and 0.9mm x 152mm)
- Depth Gauge
- Measurement Ruler
- K-Wire Exchanger
- HCS Wire Pusher
- Handle
- Countersink
- 4mm, 2.7mm, and 3.3mm Cannulated Reamer
- T8 and T10 Cannulated AO Driver
- T8 and T10 Universal QC Driver
Interested in the Threaded Hand Nail?

Founded in 2007 by Dr Jorge Orbay, MD, Skeletal Dynamics set out to provide innovative, science-based solutions to solve and understand the clinical challenges of upper extremity surgery and unmet clinical needs. Skeletal Dynamics is the only medical device company that is 100% solely focused on the upper extremities. Distributors of Skeletal Dynamics are selected based on individual integrity, industry experience and clinical knowledge and LEDA is proud to be the leading UK orthopaedic distributor for Skeletal Dynamic products including:
Frequently Asked Questions
Yes. Both the Threaded Hand Nails and the accompanying instrumentation are supplied non-sterile and must be sterilised at the user facility. The system should also be cleaned prior to sterilisation, with full instructions on the process provided with the product.
No. Prior to use, it must be checked whether the patient has osteoporosis, sepsis, an infection, insufficient bone quality/quantity, or material sensitivity. If any of these conditions are present, the nails should not be used.
There are a range of potential adverse events associated with the use of the Threaded Hand Nail system. This may include pain, swelling, stiffness, nerve/soft tissue damage. Care must be taken during the procedure the nail is not subjected to excessive force, prolonged loading, or excessive activity, as this could lead to breakage. Surgeons must evaluate the level of appropriateness for using the THN system based on their knowledge and experiences.