- 10th February 2026 12:00 am
LEDA Ortho
Established in October 2013, LEDA Orthopaedics began life as a sales agency and quickly grew into a leading orthopaedic distributor in the UK and Europe, working with innovative suppliers from all over the world. Founding directors David Plane and Jon Bloy have over 40 years’ collective experience in the orthopaedic industry and a passion for the sector that shines through in their ongoing commitment to the business to this day. Read More…
Specialist UK distributors representing:
Latest News

How to spot and treat Thumb Base Arthritis (CMC Joint Osteoarthritis)
The carpometacarpal (CMC) joint of the thumb (also called the trapeziometacarpal joint) is highly mobile and is subjected to considerable forces during pinch, grip, and dexterous tasks. This combination makes it vulnerable to degenerative changes, overuse, trauma, and instability. When conservative measures fail to deliver adequate pain relief or function, surgical intervention becomes a consideration.
Understanding when surgery is appropriate, which surgical options exist, and what outcomes to expect is essential for both patients and clinicians. In the UK, the British Society for Surgery of the Hand (BSSH) has produced the BEST guideline on thumb base osteoarthritis, which provides structured recommendations.
Below, we review:
- The pathology, symptoms, and conservative management
- Indicators and criteria for proceeding to surgery
- The main surgical options, risks, and expected outcomes
- Key counselling points and follow-up considerations
Pathology, Symptoms & Conservative Management
Pathology & Biomechanics
The thumb CMC joint sits between the base of the first metacarpal and the trapezium bone of the wrist. It allows flexion/extension, abduction/adduction, and a degree of axial rotation (opposition). Over time, or after trauma, cartilage degeneration, subchondral sclerosis, osteophyte formation, and joint subluxation may develop, leading to osteoarthritis.
Because the joint must both bear load and allow mobility, degeneration frequently ends up producing pain, instability, and functional limitation. In more advanced cases, the joint’s alignment may shift, and secondary changes (e.g., in adjacent joints) may occur.
Clinical Presentation & Diagnosis
Typical symptoms include:
- Pain at the base of the thumb (often worse during pinch or grip activities, e.g. opening jars, turning keys)
- Tenderness on palpation of the CMC joint
- Swelling, crepitus, or bony prominence at the base of the thumb (“bossing”)
- Weakness of pinch, grip fatigue
- Loss of range of motion, stiffness
- In advanced cases, deformity (a “zigzag” thumb posture) due to metacarpophalangeal hyperextension compensating for CMC collapse
On examination, doctors often test for pain with load across the joint, “grind test” (axial compression + rotation), and assess stability. Plain radiographs are standard (AP, lateral, oblique views) to grade the severity of arthritic change, subluxation, and joint space narrowing.
Where there is ambiguity about the involvement of adjacent joints (e.g. the scaphotrapeziotrapezoidal joint, STT), further imaging may be necessary.
Conservative Management (First Line)
All patients should undergo non‑surgical management first, unless the condition is extreme.
According to the BSSH BEST guideline, a stepwise multimodal approach is recommended. Common measures include:
- Education and activity modification (avoiding aggravating tasks)
- Analgesics / NSAIDs (or topical agents)
- Splinting / orthoses (thumb spica or CMC support) to offload the joint
- Hand therapy: strengthening of thenar muscles, joint protection techniques, exercises
- Intra‑articular corticosteroid injections for temporary relief in recalcitrant cases
According to the BSSH BEST guidance, if symptoms persist despite “a comprehensive non-invasive management package” (splinting, therapy, analgesics), then surgical options can be considered.
However, it is important to emphasise that conservative measures may not completely remove all symptoms but aim to improve pain control, maintain function, and delay or avoid surgery.
When Is Surgery the Right Option?
Moving to surgery is a significant decision. Not every patient with thumb CMC osteoarthritis is a surgical candidate, and not all surgical techniques are appropriate for everyone. The decision should balance symptom severity, functional limitation, patient expectations, comorbidities, and risk vs benefit.
Here are key indications, contraindications, and decision factors:
Indications for Surgery
- Failure of adequate conservative therapy
If pain, functional limitation, and reduction in quality of life persist despite a reasonable trial of nonoperative methods (often many months) - Daily activities severely impaired
If tasks such as grasping, pinching, opening jars, turning keys, or personal tasks are markedly restricted, despite nonoperative measures. - Progressive disease and structural collapse
Radiographic progression, subluxation, joint instability or deformity may push the balance toward surgical intervention if symptomatic. - Patient expectations and tolerances
Some patients may accept residual symptoms and adaptation; others may prefer more definitive surgical correction. - Good surgical candidacy
Patients who are medically fit, with realistic expectations, and able to engage with postoperative rehabilitation.
The BSSH BEST guideline suggests that if symptoms fail to resolve after non-invasive management, surgery should be considered.
Surgical Options: Techniques, Pros & Cons, Evidence
Once surgery is judged to be the right option, surgeons may choose among several techniques. The most common ones include:
- Trapeziectomy (excision of the trapezium)
- Trapeziectomy + Ligament Reconstruction / Tendon Interposition (LRTI)
- Arthrodesis (fusion) of the CMC joint
- Joint replacement / arthroplasty (total CMC prosthesis, hemiarthroplasty, or implant devices)
Each has advantages, drawbacks, and evidence.
Trapeziectomy (Simple Excision)
This is the “classic” and still most commonly used operation. It involves removing the trapezium bone to eliminate the arthritic articulation. Some surgeons may leave the gap (simple resection), while others may stabilise the thumb metacarpal using soft tissue interposition or tendon grafts.
Pros:
- Reliable pain relief in many patients
- Avoids putting a prosthesis or rigid construct
- Good long-term results in many series
- Less risk of implant-related complications (loosening, wear)
Cons:
- Potential instability or shortening of the thumb
- Loss of pinch strength compared to an ideal prosthesis
- Prolonged rehabilitation and adaptation
The BSSH BEST guideline notes that additional procedures (interposition or ligament reconstruction) do not appear to confer major benefit over simple excision (trapeziectomy alone) in their systematic evaluation.
Trapeziectomy + Ligament Reconstruction / Tendon Interposition (LRTI)
To address concerns about instability or metacarpal collapse, surgeons often pair trapeziectomy with a soft tissue procedure — e.g. using a strip of the flexor carpi radialis (FCR) tendon to reconstruct ligaments or interpose tissue in the gap (thus stabilising the thumb).
Pros:
- Additional stabilisation may better preserve pinch strength
- Less risk of metacarpal subluxation or collapse
- Many surgeons believe it yields a more stable thumb base
Cons:
- More surgical complexity
- Donor tendon morbidity
- Slightly longer recovery
- Mixed evidence on superiority over simple trapeziectomy (BSSH BEST suggests limited added benefit)
CMC Joint Fusion (Arthrodesis)
Fusion of the CMC joint is less common and is typically reserved for younger patients or in specific circumstances. By fusing the joint, pain is eliminated but mobility is sacrificed.
Pros:
- Stable, pain-free base
- May preserve grip strength better than excision in some patients
Cons:
- Loss of mobility of the CMC (no motion at that joint)
- Increased stresses on adjacent joints (e.g. MCP, wrist)
- Risk of non-union, hardware failure
Because of the mobility lost, fusion is used selectively (e.g. high demand or where implant options are not favourable).
Joint Replacement / Arthroplasty
This involves replacing the articulating surfaces with a prosthesis (either full or partial). Several designs exist: total CMC implants, hemiarthroplasty, or synthetic cartilage implants (e.g. the MAIA implant) in more recent practice.
Pros:
- Potential for better preservation of motion
- Theoretically improved function and biomechanical behaviour
- Faster return to certain tasks in some series
Cons:
- Risk of implant loosening, subsidence, failure, dislocation over time
- Revision surgery may be required
- Higher cost, greater technical demand
- Long-term durability is still under study
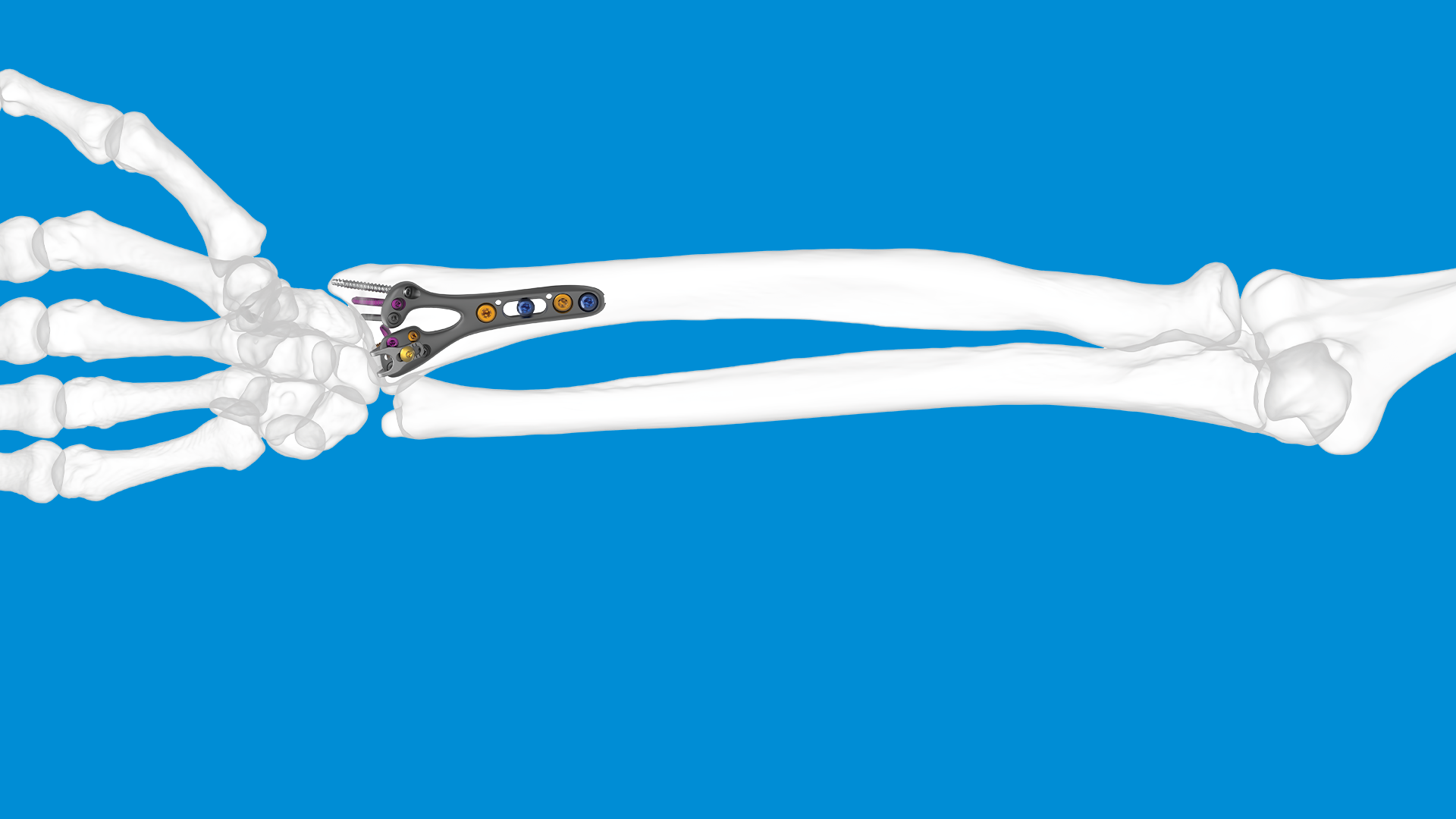
The MAIA implant could be an excellent option given its claimed advantages:
- Restores natural thumb movement using a dual-mobility ball-and-socket design.
- Improves pain, function, and grip/pinch strength compared with baseline (and often compared with trapeziectomy).
- Provides an anatomical fit through multiple cup, stem and neck size options.
- Offers stable long-term fixation via porous, cementless components and titanium options for metal-sensitive patients.
- Aims for faster recovery and an earlier return to hand function than traditional alternatives.
To find out more about MAIA, please visit our dedicated page: MAIA – CMCJ Replacement – LEDA.
One clinical series of cemented total trapeziometacarpal implants in advanced disease (Eaton stage III/IV) showed good outcomes at average 59 months: most patients were pain-free; average pinch strength ~85% of the unaffected side; minimal loosening in follow-up.
In the UK, when using implants like MAIA, aftercare protocols typically involve cast / splint immobilisation initially, followed by rehabilitation over weeks to months.
Trapeziectomy remains a gold standard in many UK services, partly because of its reliability and lower risk of long-term implant complications; the BEST guideline acknowledges prosthetic techniques but highlights the need for balanced decision-making.
Expected Outcomes, Risks & Counselling
Outcomes & Time Course
- Pain relief is the primary target. Most patients notice improvement within a few months; full recovery may take 6 to 12 months.
- Range of motion generally improves, but residual stiffness may persist.
- Pinch strength often recovers, though rarely to full pre‑disease levels, especially in heavier-demand tasks.
- Implant-based surgeries carry some probability of revision over time; prosthetic loosening or failure may appear years later.
Risks & Complications
Common risks to discuss include:
- Infection, wound healing problems
- Nerve irritation or injury (sensory branches near the thumb base)
- Tendon injury
- Persistent pain or lack of relief
- Implant failure, loosening, dislocation (for arthroplasty)
- Complex Regional Pain Syndrome (CRPS)
- Non-union (if fusion attempted)
- Loss of joint motion (especially with fusion)
- Donor tendon morbidity (in LRTI)
Shared Decision Counselling
To help patients decide, the clinician should:
- Explain the prospects vs risks of each surgical option, in light of their age, activity level, imaging findings, and expectations
- Clarify that surgery is not guaranteed to restore full strength or eliminate all symptoms, but aims to improve pain and function
- Discuss the rehabilitation commitment and timeline (often many months)
- Review alternative strategies and risks of delaying surgery (progressive joint damage, possible worsening of symptoms)
- Emphasise the importance of selecting a surgeon experienced in thumb CMC procedures and in long‑term follow-up
Practical Algorithm: From Diagnosis to Surgical Decision
Here is a simplified decision pathway:
- Diagnosis & grading
- Confirm CMC joint involvement, assess radiographs (stage disease), check adjacent joint involvement.
- Trial of nonoperative treatment (3–12 months)
- Splints, therapy, activity modification, analgesics, injections
- Reassess symptom severity & function
- If acceptable, continue nonoperative care
- If inadequate, consider surgical referral
- Patient evaluation & counselling
- Medical fitness, expectations, hand dominance, occupation
- Choose appropriate surgical technique
- For most patients, trapeziectomy (with or without LRTI)
- In select patients, arthroplasty/fusion options
- Surgery + postoperative care
- Immobilisation, hand therapy, phased rehabilitation
- Monitoring outcomes and complications
- Radiographic follow-up, functional scores, grip/pinch strength
The BSSH BEST guideline endorses a stepwise approach, whereby surgery is reserved for those who do not respond to conservative treatment.
Summary & Recommendations
- Thumb CMC (trapeziometacarpal) osteoarthritis is common and painful, often requiring a structured management plan.
- Conservative treatments (splints, therapy, analgesics, injections) are first-line and should be given a fair trial.
- Surgery becomes an option when symptoms remain debilitating, functional limitation is significant, and imaging supports structural change.
- Among surgical options, trapeziectomy (with or without ligament reconstruction / tendon interposition) remains the mainstay in many UK practices, with a good track record of pain relief and acceptable outcomes.
- Arthroplasty / joint replacement is a promising alternative in selected patients, though long-term durability and revision risk must be weighed.
- Fusion is reserved for specific scenarios and is less favoured due to loss of motion.
- Outcomes tend to improve gradually over months; patients should be counselled about realistic expectations, recovery time, and the risk of residual limitations or complications.
- Shared decision-making, skilled surgical technique, and dedicated postoperative rehabilitation are all critical to maximise results.
To explore differences in stability between a trapeziometacarpal prosthesis and trapeziectomy/ligamentoplasty, please see the clinical paper linked below:
Hyperextension MP.pdf

5 Reasons to Partner with LEDA Ortho
In today’s dynamic orthopaedic and trauma device marketplace, choosing the right distribution or commercial partner is a strategic decision. LEDA Orthopaedics has built a reputation as a responsive, clinically engaged, and quality‑driven UK orthopaedic distributor. For implant and device manufacturers, hospitals, clinical services, and surgical teams, partnering with LEDA can bring distinct advantages. Here are five compelling reasons to consider:
1. Clinically Informed, Consultative Approach
One of our differentiators is our emphasis on procedural knowledge, clinician engagement, and personalised advisory support rather than just transactional supply. LEDA is not a passive distributor: we aim to provide “consultative procedural knowledge and personal product advice” to our clientele.
In the orthopaedic field, where device selection, surgical technique, and intraoperative decision-making are interdependent, this consultative posture helps mitigate risk, improves adoption success, and builds trust with surgeon users. Many manufacturers struggle when distributors lack clinical depth; LEDA’s model helps bridge that gap.
2. Niche Focus, Flexibility & Responsiveness
LEDA operates as an independent, UK-based specialist orthopaedic distributor, established in 2013, with a focus on upper-limb, foot & ankle, trauma, and niche orthopaedics.
This relatively lean, focused approach confers several advantages:
- Faster responsiveness to market changes, surgeon feedback, and product innovation.
- Greater flexibility in customizing support, trialling new products, and adapting logistics to local needs.
- Closer alignment with customer needs (e.g. surgeon requests, niche specialties) rather than fitting into a one-size-fits-all national model.
Large distributors often have rigid systems, lengthy lead times, or diluted attention to smaller product lines. A specialist partner like LEDA can nurture emerging technologies and give them the attention they deserve.
3. Broad & Exclusive Distribution Portfolio
Partnering with LEDA provides access to a curated portfolio of orthopaedic and trauma technologies, some of which we hold exclusive distribution rights in the UK & Ireland:
- LEDA is the UK distributor for Skeletal Dynamics and Toby Ortho for upper-limb trauma solutions.
- Distributors of The Medi-X Phoenix 3000 –The Medi-X Phoenix 3000 streamlines hand surgery by replacing conventional tables with a compact, all-in-one system and integrated X-ray imaging
- We act as the UK distributor for The Lépine Group’s MAIA CMCJ prosthesis, a surgical solution for the treatment of basal thumb osteoarthritis. MAIA features a dual-mobility design for enhanced stability, modular components for anatomical positioning, and uncemented fixation supported by modern instrumentation
- Our presence across trauma, upper-limb, foot & ankle, and imaging gives manufacturers access to multiple overlapping clinical verticals through a single partner.
For a device manufacturer, this breadth means less fragmentation of sales channels and consistency in representation across hospital specialties. For clinical customers, it means more synergy and convenience from having a trusted vendor across multiple product lines.
4. Aligned with Quality, Standards & Ethical Practice
Any credible partner in the UK orthopaedics space must align with standards of quality, transparency, and ethics. Our public profile and activities suggest we take this alignment seriously:
- Our role as an exhibitor and technology partner at BOA Congress (notably with the HiRise scanner) demonstrates our engagement with the orthopaedic professional community.
- Our consultative and clinically grounded model helps ensure the products implemented are fit for purpose, thereby supporting high-quality patient care — a priority consistent with the BOA’s mission to advance orthopaedic practice.
- Ethical collaborations matter: the BOA’s Code of Ethics stipulates that relationships between clinicians and industry must be transparent, proportionate, and free from undue influence.
- Because LEDA is comparatively smaller and more focused, there is greater clarity and accountability in each partnership, which reduces risk in meeting governance and regulatory expectations.
For manufacturers and clinicians, partnering with an entity that understands and respects the demands of audit, regulatory compliance, and conflict-of-interest transparency is a strong safeguard.
5. Growth-Oriented Partnerships & Innovation Support
We are not just a distributor; we position ourselves as partners in innovation and growth.
For example:
- LEDA has recently entered a partnership to distribute MY01’s continuous compartment pressure monitoring technology in the UK. This move demonstrates our willingness to adopt cutting-edge medical devices and support our integration into clinical practice.
- Our model includes training, procedural support, and education, which are essential for new device adoption. Manufacturers often fail when distribution is purely transactional without clinical training or support — LEDA’s model addresses that gap.
- We have shown capability to expand our distribution network. In past collaborations (e.g. with Surgical Holdings), LEDA was appointed to distribute orthopaedic sets in new regions, showing they can scale distribution responsibilities.
- Because we work across multiple specialties (upper limb, imaging, trauma), we are well placed to cross-promote and enable synergistic solutions (e.g. combining imaging + implant + fixation).
If a manufacturer or innovator wants a UK foothold, LEDA can act as a conduit for clinical trials, key opinion leader relationships, registry data collection, and surgeon engagement.
Putting It into Practice: What a Partnership with LEDA Can Achieve
To illustrate the synergy, here’s how a hypothetical collaboration might look:
- Product Launch & Surgeon Engagement
A manufacturer of a new mini-implant system partners with LEDA. LEDA arranges surgeon workshops, cadaver labs, and hospital adoption pilots, leveraging its clinical relationships and procedural support. - Logistics & Supply Chain
LEDA coordinates inventory in UK distribution centres, ensures regulatory compliance (CE / UKCA), and optimises responsiveness to hospital demands—reducing lead times and stockouts. - Clinical Support & Feedback Loop
Post-launch, LEDA collects surgeon feedback, complication or performance data, and helps refine product iterations. This feedback loop helps improve quality and clinician satisfaction. - Synergistic Bundling
Because LEDA also distributes compatible imaging and orthopaedic systems (e.g. Phoenix 3000), we may bundle product offerings or promote usage pathways (e.g. imaging + fixation) - Compliance & Ethical Oversight
All interactions, training, and promotional activity follow transparent protocols, conflict-of-interest disclosures, and proportional clinical engagement—ensuring alignment with BOA’s Code of Ethics.
Over time, this approach helps build trust, drive adoption, reduce friction in procurement and clinical integration, and ultimately improve patient outcomes.
Summary & Invitation to Collaborate
In summary, here are the five key reasons to partner with LEDA Ortho:
- Clinically informed, consultative approach that safeguards adoption quality
- Niche focus, flexibility & responsiveness in a crowded distributor landscape
- Broad, exclusive distribution reach across trauma, upper limb, fixation, and imaging
- Alignment with quality, standards & ethical practice expected by professional bodies
- Growth-oriented partnership & innovation support for new technologies
If your organisation is evaluating UK distribution partners, or you are seeking a more clinically engaged, responsive, and quality-aligned collaborator, LEDA Ortho presents a compelling option. Please contact us for more information, call +44 (0) 1480 457222 or email sales@ledaortho.com.

The role of radial head replacement in complex elbow injuries
The radial head is a critical component of elbow anatomy contributing to stability, load transmission, and forearm rotation. In complex elbow injuries — such as comminuted radial head fractures, fracture‑dislocations of the elbow (e.g. “terrible triad” injuries), or associated ligamentous damage — the normal anatomy and biomechanical stability are disrupted. In such settings, radial head replacement (sometimes called radial head arthroplasty, RHA, or prosthetic replacement) has emerged as a key surgical option alongside other strategies like open reduction internal fixation (ORIF), resection, ligament repair, or combinations thereof.
Anatomy, Biomechanics & Types of Injury
To appreciate when and why radial head replacement is used, it’s useful to recall what role the radial head plays:
- It acts as a secondary stabiliser against valgus stress, especially when the medial collateral ligament (MCL) is damaged.
- It helps resist axial (longitudinal) load and contributes to stability in posterolateral rotatory injuries.
- In “terrible triad” patterns (radial head fracture + coronoid fracture + elbow dislocation) and other fracture‑dislocations, the radial head is usually irreparably damaged or contributes significantly to joint instability.
The Mason classification (modified) is often used to classify radial head fractures. Mason type III (comminuted fractures) or type IV (fractures with dislocation) are more severe and frequently where replacement becomes a serious option.
Indications for Radial Head Replacement
From the literature, the main indications are:
- Unreconstructable fracture of the radial head
When there are more than three fracture fragments, severe comminution, or the bone quality is poor, making ORIF unlikely to succeed. - Associated instability
If there is ligamentous injury (lateral collateral ligament [LCL], MCL), dislocation, a coronoid process fracture, or other soft tissue damage, then maintaining stability becomes crucial. The radial head helps restore stability in valgus, posterolateral rotatory forces, etc. If the radial head is damaged and cannot be fixed, then replacement helps restore the lateral column and joint congruence. - Fracture dislocations and “terrible triad” injuries
In these, surgeons frequently decide in favour of replacement over fixation if the radial head is too badly damaged or reconstructable only with high risk. Early surgical intervention is associated with better outcomes. - When ORIF would likely have a high complication rate
For example non‑union, hardware failure, secondary surgeries, inability to restore anatomy, risk of stiffness, etc. Some studies comparing ORIF vs RHA in complex patterns find better functional outcomes, less reoperation with replacement in appropriately selected patients.
Techniques and Considerations
Using radial head replacement successfully involves careful attention to multiple surgical and implant‑based details:
- Implant design: Modular vs monoblock, stem type (press-fit, cemented), head diameter and height, smooth vs rough stems. Some recent literature shows that “loose‑fit, polished stem” prostheses may perform well in complex injuries, possibly with less stress at the bone‑implant interface and fewer problems of loosening.
- Size & height of prosthesis: Overstuffing (implant too large or positioned too high) may lead to capitellar overload, pain, stiffness. Under sizing or undersetting may fail to restore stability. The exact anatomical matching is important.
- Timing of surgery: Early repair or replacement (ideally within 1–3 weeks) tends to produce better outcomes in many series. Delayed surgery is associated with worse function and more complications.
- Addressing associated injuries: Besides the radial head, repair of coronoid process, stabilisation of collateral ligaments (especially lateral), sometimes medial if needed. Often the elbow will remain unstable unless all major components are addressed.
- Postoperative rehabilitation: Early mobilisation, physiotherapy, to reduce stiffness. However, must balance with protection of repairs. Also keeping an eye on potential complications (nerve injury, heterotopic ossification etc.).
Outcomes: What Does the Evidence Say?
Here are some key findings from recent studies:
- A study of longer‑term outcomes of radial head arthroplasty for complex elbow fracture‑dislocations (using modular monopolar prostheses) showed decent functional results >10 years, though periprosthetic radiolucency (indicator of bone‑implant interface changes) was noted, and component removals sometimes needed.
- In systematic reviews comparing radial head replacement (RHA) vs reconstruction (ORIF/repair) in “terrible triad” injuries, RHA often shows better range of motion, higher functional scores (MEPS, DASH) and fewer reoperations/complications in those injuries where the radial head is irreparably damaged.
- Implant survival: One study with monopolar radial head prostheses had implant survival ~75.1% at 18 years, though early reoperation/removal risk was high (mostly within the first postoperative year).
- Another recent cohort showed that although RHA is associated with a relatively high risk of reoperation (about 25% in one large acute fracture group), the functional outcomes (QuickDASH, Oxford Elbow Score) remain good for many, and many reoperations are within the first 12 months.
- A study of the “surgical treatment of the radial head” as part of terrible triad injuries (88 patients, mean 4.5 years follow-up) showed that using ORIF for reconstructable fractures and RHA when reconstruction was not feasible achieved good average functional scores (MEPS ~87, OES ~37, DASH ~19) in those cohorts.
Risks, Limitations, and Patient Counselling
While RHA offers many advantages, several important limitations, risks, and trade‑offs must be discussed with patients.
- High early reoperation / removal rates: Many failures, revisions, or removals occur in the first year. Surgeons must warn patients of this.
- Long‑term concerns: Loosening, wear, periprosthetic bone changes, possibility of needing future surgery, risk of arthritis or capitellar overload if sizing/positioning is suboptimal.
- Functional compromise: Even with “successful” operations, full restoration of motion is rarely perfect. Loss especially of extension, supination/pronation may persist. There may be residual pain.
- Soft tissue injury outcomes: Even a well‑implanted radial head prosthesis cannot fully compensate if ligaments (especially collateral ligaments) or coronoid fractures are not addressed. Stability depends on a holistic reconstruction.
- Age, patient demand, comorbidities: Younger patients may be more concerned about long‑term durability; patients with poor bone stock or general health issues may have increased risk of complications.
UK Context & Practice Patterns / Guidance
In the UK, specific BOA guidelines on radial head replacement are not, at least publicly, as detailed as for some other orthopaedic problems. Still:
- The National Joint Registry in the UK lists “radial head replacement” as one of the elbow replacement procedures. The patient information materials describe that radial head replacement involves a short stem and head to replace the top end of the radius and note that implants of different sizes are available to match anatomy.
- NHS patient information leaflets for radial head/neck fractures typically describe that simple fractures are treated non‑operatively, while displaced, comminuted, or those causing mechanical block or instability may require surgery. Radial head replacement is mentioned as one surgical option when fixation is not possible.
- AO Foundation / SurgeryReference guidance (used in UK and internationally) generally indicates that for complex elbow injuries (terrible triad, elbow dislocation with radial head fracture), the first surgical step is to determine if the radial head is reconstructable; if not, replacement is recommended.
Thus, while BOA doesn’t yet have a “definitive guideline” specifically naming every indication of radial head replacement, UK practice tends to follow the international evidence and these intra‑UK sources.
Algorithm / Decision‑Making Summary
Putting together where the evidence supports it, the decision to use radial head replacement in complex elbow injury might follow roughly this pattern:
| Factor | Favors ORIF / Reconstruction | Favors Radial Head Replacement |
| Fracture pattern | ≤ 2 fragments, good articular surface, bone stock good | >3 fragments, comminution, missing articular surface, poor bone quality |
| Associated injuries | Minimal, stable elbow; ligament injury well preserved | Coronoid fracture, dislocation, LCL (+/‑ MCL) injury, elbow unstable |
| Soft tissue status & timing | Early, minimal soft‑tissue damage; early surgery possible | Delayed presentation; risk of stiffness; high‑energy injury; swelling |
| Patient factors | Young, high demand, expectations of long life in prosthesis | Older, lower demand; but needs vs risk of reoperation must be balanced |
| Surgeon & implant factors | Surgeon’s experience in ORIF; access to implants; expected outcomes | Implant type available; ability to restore size/height; repair ligaments appropriately; rehab resources |
Summary: Role of Radial Head Replacement
Putting it all together, radial head replacement plays a central role in many complex elbow injuries. Key take‑home points:
- Pillar of stability: RHA is often essential to restore elbow stability in the face of severe fractures and ligament injury. Without it, there is high risk of valgus instability, subluxation, or recurrent dislocation.
- Functional outcomes are good in many cases provided reconstruction is done appropriately, implants are well chosen, and associated injuries are addressed. Even if perfect ROM is not regained, many patients report good to excellent scores on MEPS, DASH, Oxford scores.
- High risk of early complications / reoperations must be acknowledged. Patient counselling is critical. The majority of reoperations happen within 12 months. Longer‑term survivorship of well‑implanted prostheses tends to be favourable (many reports show good survival at 10‑15+ years) but subject to caveats.
- Not always the best choice: If fractures are reconstructable, ORIF may allow preservation of native bone and possibly fewer long‑term issues; for less severe injury patterns, ORIF still has a role. Also, replacement does not remove the need to address soft tissue injuries.
Unanswered Questions & Areas for Further Research
There remain gaps in the evidence, which mean that practice is still evolving:
- BOA‑level consensus or guideline specifically on radial head replacement in complex elbow injuries (e.g. definitions, implant types, thresholds for choosing replacement vs repair) is still lacking or not widely published.
- Comparative RCTs of ORIF vs RHA in particular injury subgroups (e.g. certain ages, levels of comminution, ligament injury severity) are still relatively few.
- Optimal implant design parameters (stem type, head height, modularity), and their biomechanical implications for wearer outcomes, over decades.
- Long‑term studies in UK populations, including cost‑effectiveness, rehabilitation protocols, and patient‑reported outcome measures over long follow‑up.
Clinical Implications and Recommendations
From this review, some suggested practice recommendations (based on the best available evidence) include:
- In complex elbow injuries involving severe radial head fracture plus soft tissue damage (ligaments, coronoid), strongly consider radial head replacement rather than resection or attempted fixation when reconstruction is unlikely to restore anatomy or stability.
- When replacing, choose implants that allow accurate matching of head size and height; avoid over‑ or undersizing; prefer designs shown to have lower rates of loosening, with smooth or polished stems where evidence supports them.
- Perform surgery as early as feasible once swelling permits, to reduce risk of stiffness and improve functional outcomes.
- Repair associated injuries (ligaments, coronoid) to restore overall elbow stability rather than relying solely on the radial head replacement.
- Plan for and counsel about rehabilitation: early motion, careful physiotherapy, monitoring for complications (heterotopic ossification, nerve issues, loosening).
- Clear patient counselling: expectations (range of motion, pain, need for follow‑ups, possible additional surgery), risks and benefits.
Conclusion
Radial head replacement has become a mainstay in the management of complex elbow injuries — especially for severely comminuted fractures and associated instability (e.g. terrible triad). The evidence (albeit not always from large RCTs) suggests that when used appropriately, it restores stability, yields good functional outcomes, and offers better performance than fixation or excision alone in many settings. However, it is not a panacea: surgical technique, implant choice, patient selection, timing, and managing associated injuries all highly influence outcomes.
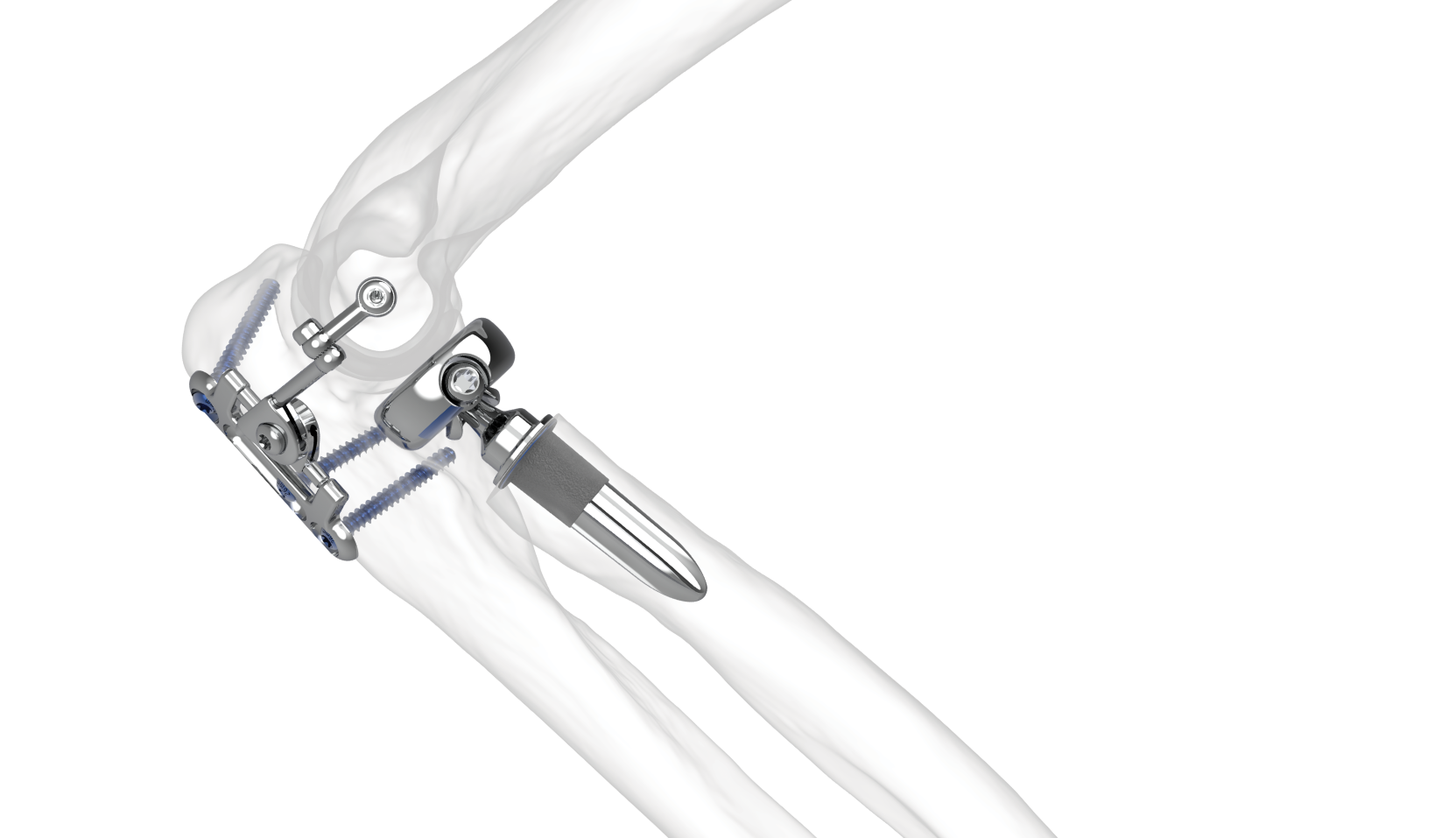
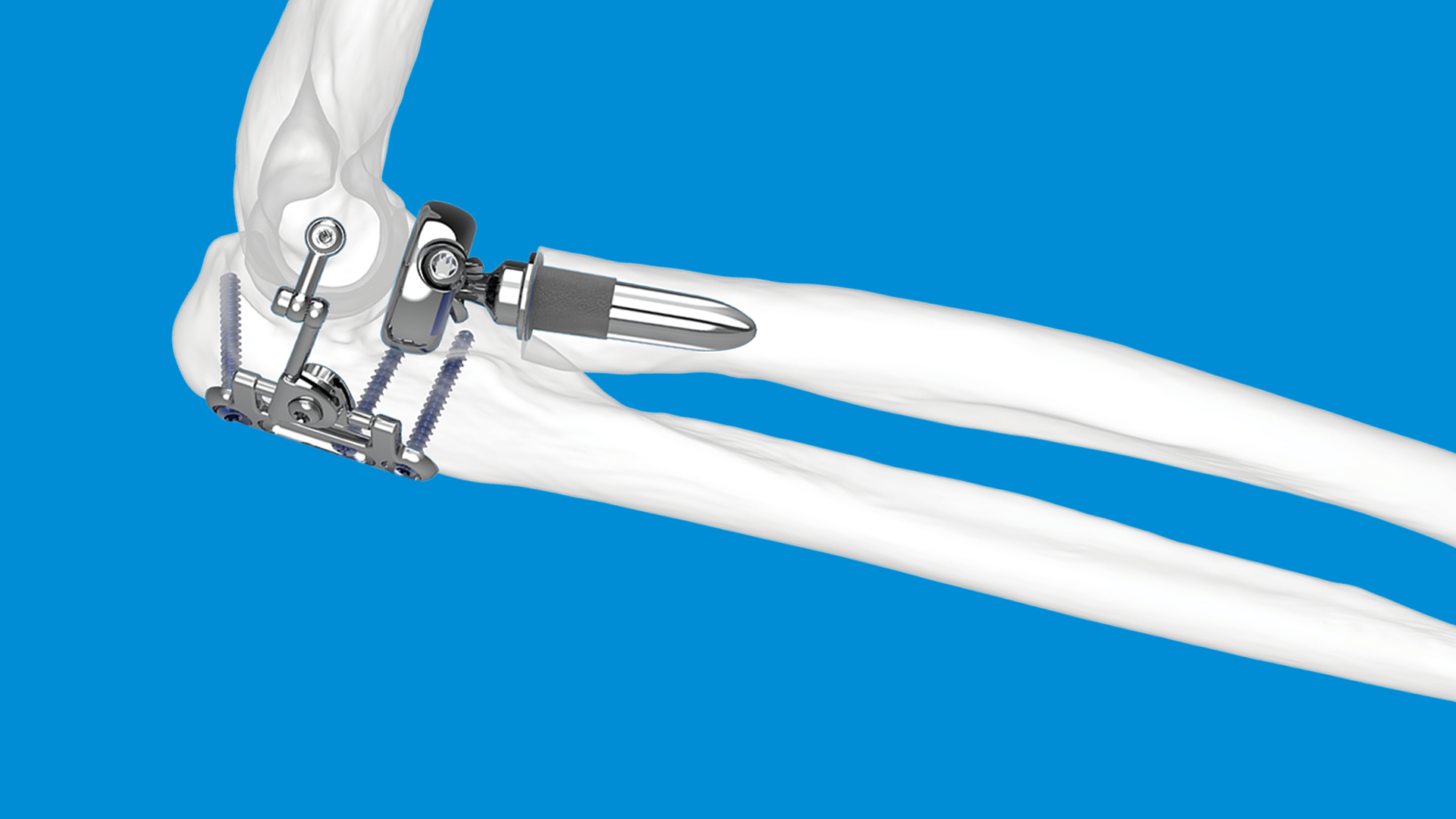
In this context, the Skeletal Dynamics ALIGN Radial Head System offers a modern, anatomically driven solution designed to support more predictable and stable outcomes. Its features reflect current thinking in elbow biomechanics and prosthetic design, including:
- An anatomically aligned monoblock head, shaped to better match native patient anatomy.
- A side-loading modular system with a broad selection of stem and neck sizes, allowing surgeons to fine-tune fit and restore joint mechanics more accurately.
- A long, press-fit stem supported by strong clinical results, helping ensure secure fixation without the need for cement.
- Digital stem flutes engineered to improve rotational stability and facilitate a more natural range of forearm motion.
Together, these design elements aim to replicate native kinematics while offering the durability and stability required in complex elbow reconstructions.
If you think a radial head replacement might be indicated in your case, you may wish to explore ALIGN via our website ALIGN – Radial Head Replacement – LEDA
Take a moment to read this study on how the Skeletal Dynamics MAIA prosthesis, designed to align with the forearm’s axis of rotation, may improve patient outcomes: A radial head prosthesis that aligns with the forearm axis of rotation: a retrospective multicenter study
The Founders