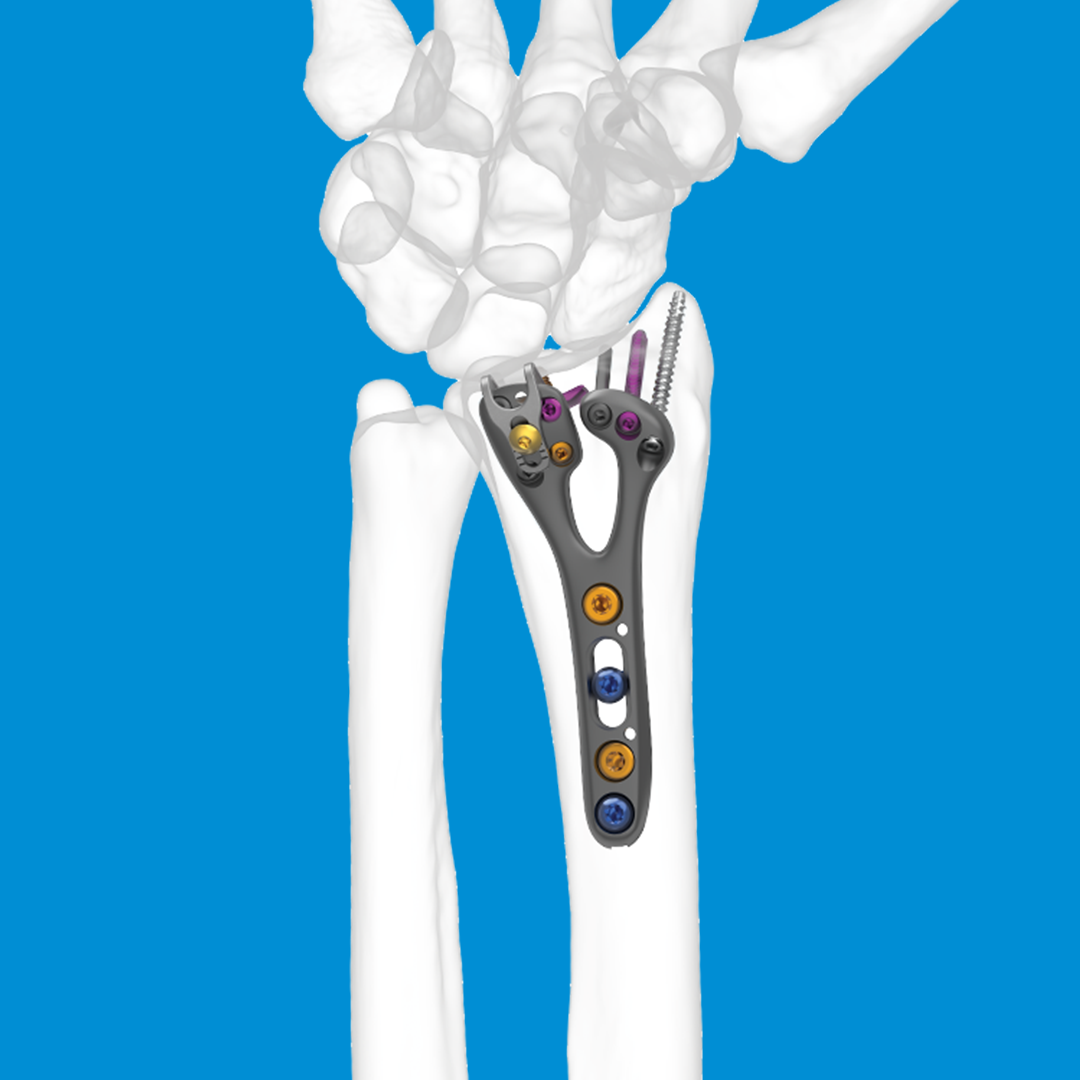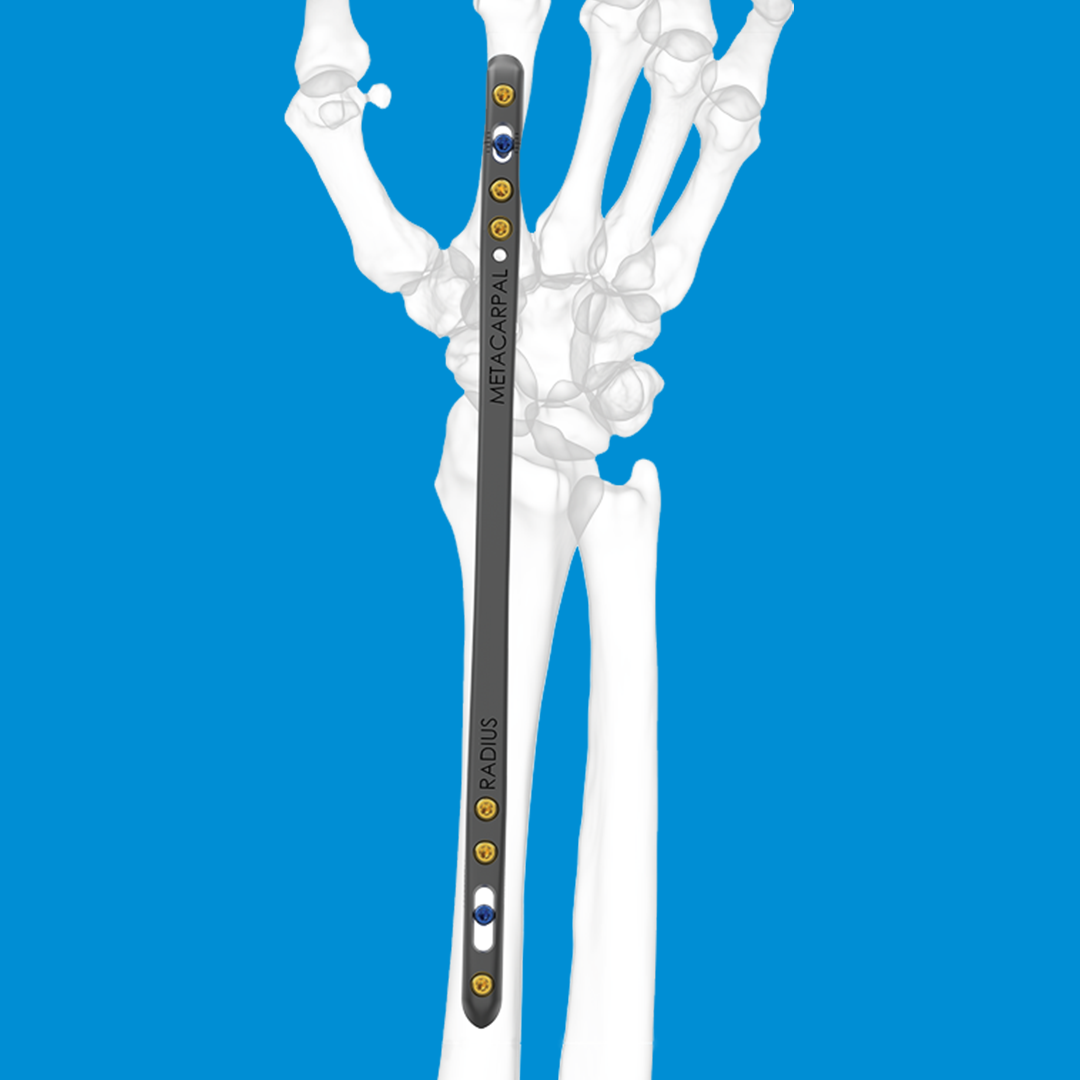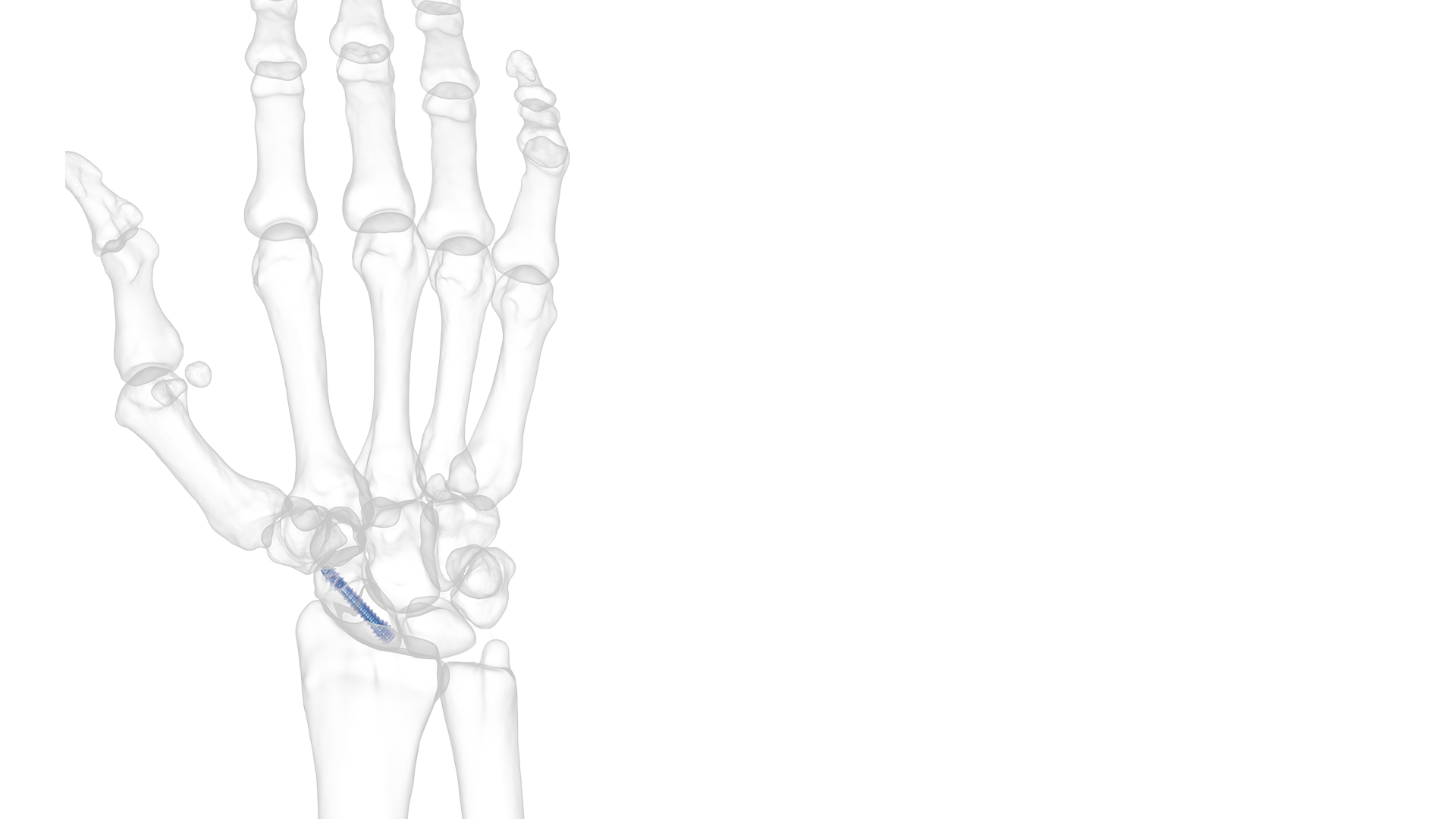
REDUCT® Headless Compression Screw System
Features
- Canulated controlled compression
- 2.5mm and 3.5mm diameters
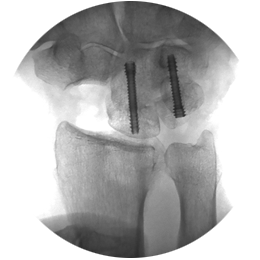
- Precise fixation of radial head and coronoid fragments
REDUCT®
The REDUCT® headless compression screw system is designed for the fixation of osseous fragments or fractures, arthrodesis of small joints, and osteotomies in the hand and wrist. Screws are sized according to patient anatomy and the specific treatment required. Common injuries treated with this system include:
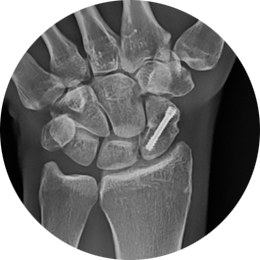
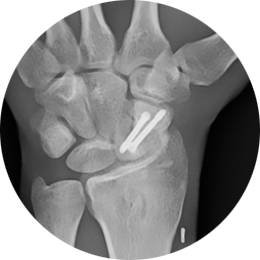
- Scaphoid fractures
- Lunate fractures
- Capitate fractures
- Trapezium fractures
- Metacarpal fractures
- Phalangeal fractures (proximal, middle, distal)
- Non-unions of carpal bones
- Interphalangeal fractures
- Carpometacarpal joint fusions
- Osteo-chondral fractures
- Distal radius fractures
- Osteotomies for metacarpals and phalanges
- Tendon insertions and small joint arthrodesis (e.g., PIP, DIP joints)
The REDUCT® Headless Compression Screw System
The REDUCT® system is designed for maximum initial contact and surface area at the screw-bone interface. The screw’s headless design allows for smooth placement without leaving any surface prominence, ideal for the intricate anatomy of the hand and wrist. For example, the REDUCT® 3.5mm screw features a leading end of 3.4mm and a trailing end of 4mm, while the 2.5mm screw has a leading end of 2.6mm and a trailing end of 3.2mm.
This design helps distribute and dissipate mechanical loads at the fracture site, facilitating bone healing and stability. Orthopaedic screws like the REDUCT® are commonly used to treat fractures by holding bone fragments in place without creating surface prominence, allowing the bone fragments to heal securely.
The compression achieved through the system is crucial for long-term fixation, ensuring that the fragments remain stable during the healing process. These screws are often left in place post-surgery because they are made from biocompatible materials that are unlikely to cause irritation or harm to surrounding tissues. Cannulated screws also help reduce soft tissue irritation, making the rehabilitation process less invasive and quicker.
System Components:
- 2.5mm and 3.5mm width cannulated titanium screws
- Specialised instrumentation including:
- Depth gauge
- Drills (Quick Connect 1.9mm and 2.7mm, Countersink 2.7mm and 3.5mm)
- K-wire (1.4mm x 165mm, 0.9mm x 152mm)
- Driver
- Both 2.5mm and 3.5mm screws are available in 11 different lengths, ranging from 10mm to 30mm in 2mm increments.
Stages of Headless Compression Screw Insertion:
- Distal fragment engaged with distal threads.
- Thread relief clears the fracture site.
- Central clutch section provides translation and compression between bone fragments.
- Slippage prevention ensures that over-compression does not occur.
- Proximal threads engage the proximal fragment, resulting in 1mm of final compression.
Through internal testing, compressive force significantly increases from stage 3 onwards. It is recommended to pre-drill the bone to optimise screw placement and avoid potential complications from axial forces that could reduce compression.
Interested in the REDUCT® Headless Compression Screw System?
If you would like more information regarding Skeletal Dynamics‘ REDUCT® Headless Compression Screw System for use in the hand and wrist, please contact us.
Interested in the REDUCT® Headless Compression Screw System ?
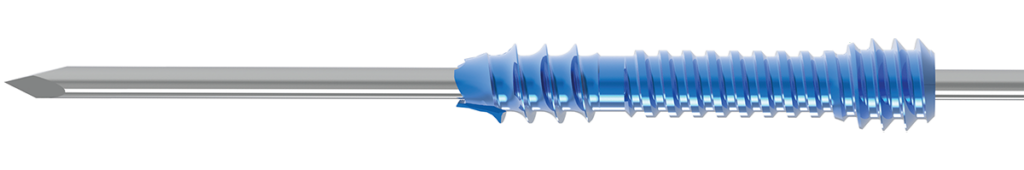
Provides the needed fragment approximation and only 1mm of reproducible compression

Founded in 2007 by Dr Jorge Orbay, MD, Skeletal Dynamics set out to provide innovative, science-based solutions to solve and understand the clinical challenges of upper extremity surgery and unmet clinical needs. Skeletal Dynamics is the only medical device company that is 100% solely focused on the upper extremities. Distributors of Skeletal Dynamics are selected based on individual integrity, industry experience and clinical knowledge and LEDA is proud to be the leading UK orthopaedic distributor for Skeletal Dynamic products including:
Frequently Asked Questions
An osseous fragment is identified as a radiodense piece of material found with a joint. They typically have sharp edges which can create complications in patients like chondral damage and eventually osteoarthritis. Osseous fragments often appear in cases of fragmented fractures. This is where a section of bone gets cracked but remains partially joined.
An osteotomy is a surgical procedure to shorten, lengthen or alter the alignment of a bone. It involves the cutting and reforming of bones. It is commonly used as an arthritis treatment or to straighten a bone that has healed abnormally following a fracture.
The scaphoid bone, located at the base of the radius, coordinates wrist motion and other hand bone position. If a scaphoid fracture is left untreated, it can heal incorrectly and create lasting wrist problems. Symptoms of a scaphoid fracture include stiffness and swelling in the wrist, redness around the wrist, pain on the thumb side of the wrist during use or after a fall on an outstretched hand. It can be months or years after an injury before arthritic pain sets in.
When the system isn’t in use, it should be stored in a cool dry place away from direct sunlight. Prior to this, it should be cleaned and disinfected within the Sterilization Tray that’s provided with the system. Steam sterilization following the ANSI/AAMI ST79:2006 guidelines is recommended. This can be accomplished at a healthcare facility using either the Pre-Vacuum Autoclave or Gravity Autoclave cycle. Flash sterilization is not advisable however, if chosen, it should be done following ANSI/AAMI ST79:2006 requirements.
Before every use, the system should be inspected accordingly for serviceability. This will encompass equipment inventory and damage, as well as required cleaning.